Síntesis y caracterización de dos materiales azo: Comparación entre un compuesto sulfonado y su análogo no sulfonado
DOI:
https://doi.org/10.36829/63CTS.v11i1.1596Palabras clave:
Grupo sulfónico, histéresis, compuesto azo, degradación térmicaResumen
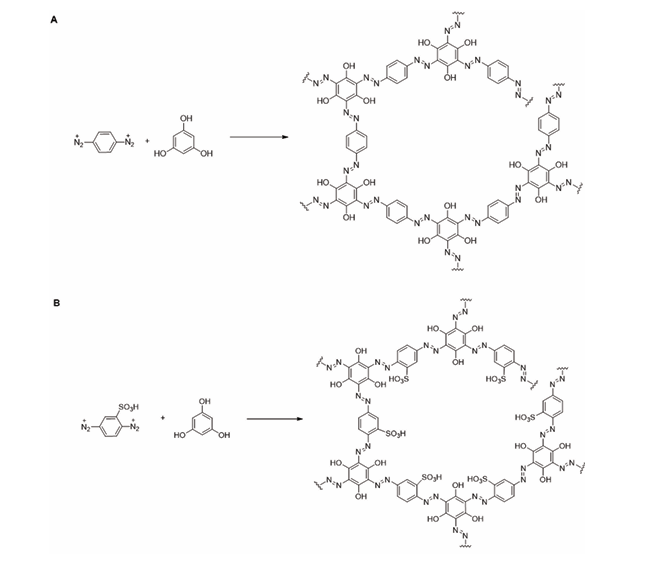
Esta investigación tuvo como objetivo la síntesis y caracterización de dos nuevos materiales azo obtenidos mediante una reacción clásica de diazotización. El primer material (A1) se sintetizó a partir de la reacción entre fluoroglucinol con p-fenilendiamina, mientras que el segundo (A2) se obtuvo a partir de la reacción entre fluoroglucinol con ácido 2,5-diaminobencensulfónico. Estos materiales fueron caracterizados químicamente mediante espectroscopia infrarroja (FTIR-ATR). Adicionalmente, se evaluó su estabilidad térmica por medio análisis termogravimétrico (TGA) y su estructura cristalina, utilizando difracción de rayos X de polvos (XRD). Los resultados muestran que A2 posee una mayor capacidad de absorción de agua atribuible a los grupos sulfónicos. Además, ambos materiales empiezan a degradarse a partir de los 140 °C y son de naturaleza amorfa, conun área de superficie menor a 1 m2g-1. Destaca que A2 posee hasta un orden de magnitud mayor que A1 para la absorción de N2. Ambos materiales mostraron características de adsorción de nitrógeno de isoterma tipo III, lo que sugiere bajas energías de interacción y propiedades de materiales no porosos.
Descargas
Citas
Ansari, M., Alam, A., Bera, R., Hassan, A., Goswami, S., & Das, N. (2020). Synthesis, characterization and adsorption studies of a novel triptycene based hydroxyl azo- nanoporous polymer for environmental remediation. Journal of Environmental Chemical Engineering, 8(2), Artículo 103558. https://doi.org/10.1016/j.jece.2019.103558
Avilés-Barreto, S. L., & Suleiman, D. (2013). Transport properties of sulfonated poly (styrene-isobutylene-styrene) membranes with counter-ion substitution. Journal of Applied Polymer Science, 129(4), 2294-2304. https://doi.org/10.1002/app.38952
Awad, A. M., Jalab, R., Benamor, A., Nasser, M. S., Ba-Abbad, M. M., El-Naas, M., & Mohammad, A. W. (2020). Adsorption of organic pollutants by nanomaterial-based adsorbents: An overview. Journal of Molecular Liquids, 301, Artículo 112335. https://doi.org/10.1016/j.molliq.2019.112335
Braun, D. E., Tocher, D. A., Price, S. L., & Griesser, U. J. (2012). The Complexity of Hydration of Phloroglucinol: A Comprehensive Structural and Thermodynamic Characterization. The Journal of Physical Chemistry B, 116(13), 3961-3972. https://doi.org/10.1021/jp211948q
Dong, S., Rene, E. R., Zhao, L., Xiaoxiu, L., & Ma, W. (2022). Design and preparation of functional azo linked polymers for the adsorptive removal of bisphenol A from water: Performance and analysis of the mechanism. Environmental Research, 206, 112601. https://doi.org/10.1016/j.envres.2021.112601
Fröschl, T., Hörmann, U., Kubiak, P., Kučerová, G., Pfanzelt, M., Weiss, C. K., Behm, R. J., Hüsing, N., Kaiser, U., Landfester, K., & Wohlfahrt-Mehrens, M. (2012). High surface area crystalline titanium dioxide: Potential and limits in electrochemical energy storage and catalysis. Chemical Society Reviews, 41(15), 5313-5360. https://doi.org/10.1039/C2CS35013K
Guerrero-Gutiérrez, E. M. A. (2021). Effect of Sulfonated Block Copolymer on the Equilibrium and Thermal Properties of Sulfonated Fluoro-block Copolymer Blend Membranes. Ciencia, Tecnología y Salud, 8(1), 57-66. https://doi.org/10.36829/63CTS.v8i1.887
Guerrero-Gutiérrez, E. M. A., Abad, M., Gaitán, I., & Guerrero, K. (2022). Efecto de las membranas con Cu+2 sobre el proceso de filtración y capacidad de biocida contra Escherichia coli. Ciencia, Tecnología y Salud, 9(1), 98-115. https://doi.org/10.36829/63CTS.v9i1.1041
Ho, M. S., Barrett, C., Paterson, J., Esteghamatian, M., Natansohn, A., & Rochon, P. (1996). Synthesis and Optical Properties of Poly{(4-nitrophenyl)[3-[N-[2-(methacryloyloxy)ethyl]-carbazolyl]] diazene}. Macromolecules, 29(13), 4613-4618. https://doi.org/10.1021/ma951432a
Huang, X.-Q., Hong, X., Lin, H., Cao, X.-M., Dang, Q., Tang, S.-B., Chen, D.-L., & Zhang, Y. (2022). Hypercrosslinked triazine-phloroglucinol hierarchical porous polymers for the effective removal of organic micropollutants. Chemical Engineering Journal, 435(Part 2), Artículo 134990. https://doi.org/10.1016/j.cej.2022.134990
Hung, L.-C., & Pan, N.-H. (2023). Using thermal analysis with kinetic calculation method to assess the thermal stability of azo compound on construction materials. Journal of Loss Prevention in the Process Industries, 84, Artículo 105107. https://doi.org/10.1016/j.jlp.2023.105107
Kazem-Rostami, M. (2020). Factors influencing the thermal stability of azo and bisazo compounds. Journal of Thermal Analysis and Calorimetry, 140(2), 613-623. https://doi.org/10.1007/s10973-019-08884-4
Kim, D.-J., & Nam, S.-Y. (2014). Characterization of Sulfonated Silica Nanocomposite Electrolyte Membranes for Fuel Cell. Journal of Nanoscience and Nanotechnology, 14(12), 8961-8963. https://doi.org/10.1166/jnn.2014.10073
Li, H.-Q., Liu, X., Zhang, Q., Li, S.-S., Liu, Y.-M., He, H.-Y., & Cao, Y. (2015). Deoxygenative coupling of nitroarenes for the synthesis of aromatic azo compounds with CO using supported gold catalysts. Chemical Communications, 51(56), 11217-11220. https://doi.org/10.1039/C5CC03134F
Li, Y., Xie, M., Wang, X., Chao, D., Liu, X., & Wang, X. (2013). Novel branched sulfonated poly(ether ether ketone) membranes for direct methanol fuel cells. International Journal of Hydrogen Energy, 38, 12051-12059. https://doi.org/10.1016/j.ijhydene.2013.06.090
López-Pardo, G. E. A., García-Guerra, C. A., Lainfiesta, R., & Guerrero-Gutiérrez, E. M. A. (2022). Caracterización colorimétrica del proceso termogravimétrico de la deshidroxilación de caolín hidrotermal y de toba. Ciencia, Tecnología y Salud, 9(1), 55-69. https://doi.org/10.36829/63CTS.v9i1.924
Lorenzo, M., Campo, J., & Picó, Y. (2018). Analytical challenges to determine emerging persistent organic pollutants in aquatic ecosystems. TrAC Trends in Analytical Chemistry, 103, 137-155. https://doi.org/10.1016/j.trac.2018.04.003
Lu, J., & Zhang, J. (2014). Facile synthesis of azo-linked porous organic frameworks via reductive homocoupling for selective CO2 capture. Journal of Materials Chemistry A, 2(34), 13831-13834. https://doi.org/10.1039/C4TA03015J
Luo, X.-S., Deng, H.-L., Chi, S., Liu, Y., & Huang, M.-H. (2021). 15N Solid-State NMR as Bright Eyes to See the Isomerization of the Azo Bond: Revision of Tris(β-hydroxyl-azo)benzene to Tris(β-keto-hydrazo)-cyclohexane in Porous Organic Polymers. The Journal of Physical Chemistry Letters, 12(29), 6767-6772. https://doi.org/10.1021/acs.jpclett.1c01750
Mandal, J., Jia, M., Overvig, A., Fu, Y., Che, E., Yu, N., & Yang, Y. (2019). Porous Polymers with Switchable Optical Transmittance for Optical and Thermal Regulation. Joule, 3(12), 3088-3099. https://doi.org/10.1016/j.joule.2019.09.016
Meyers, M. A., Mishra, A., & Benson, D. J. (2006). Mechanical properties of nanocrystalline materials. Progress in Materials Science, 51(4), 427-556. https://doi.org/10.1016/j.pmatsci.2005.08.003
Murad, A. R., Iraqi, A., Aziz, S. B., N. Abdullah, S., & Brza, M. A. (2020). Conducting polymers for optoelectronic devices and organic solar cells: A review. Polymers, 12(11), Artículo 2627. https://doi.org/10.3390/polym12112627
Peplowski, L., Szczesny, R., Skowronski, L., Krupka, A., Smokal, V., & Derkowska-Zielinska, B. (2022). Vibrational spectroscopy studies of methacrylic polymers containing heterocyclic azo dyes. Vibrational Spectroscopy, 120, Artículo 103377. https://doi.org/10.1016/j.vibspec.2022.103377
Pomonis, P. J., Petrakis, D. E., Ladavos, A. K., Kolonia, K. M., Armatas, G. S., Sklari, S. D., Dragani, P. C., Zarlaha, A., Stathopoulos, V. N., & Sdoukos, A. T. (2004). A novel method for estimating the C-values of the BET equation in the whole range. Microporous and Mesoporous Materials, 69(1), 97-107. https://doi.org/10.1016/j.micromeso.2004.01.009
Sánchez-Jiménez, P. E., Pérez-Maqueda, L. A., Perejón, A., & Criado, J. M. (2009). Combined kinetic analysis of thermal degradation of polymeric materials under any thermal pathway. Polymer Degradation and Stability, 94(11), 2079-2085. https://doi.org/10.1016/j.polymdegradstab.2009.07.006
Sava, I., Burescu, A., Stoica, I., Musteata, V., Cristea, M., Mihaila, I., Pohoata, V., & Topala, I. (2015). Properties of some azo-copolyimide thin films used in the formation of photoinduced surface relief gratings. RSC Advances, 5(14), 10125-10133. https://doi.org/10.1039/C4RA14218G
Sava, I., Hurduc, N., Sacarescu, L., Apostol, I., & Damian, V. (2013). Study of the nanostructuration capacity of some azopolymers with rigid or flexible chains. High Performance Polymers, 25(1), 13-24. https://doi.org/10.1177/0954008312454151
Selivanova, G. A. (2021). Azo chromophores for nonlinear-optical application. Russian Chemical Bulletin, 70(2), 213-238. https://doi.org/10.1007/s11172-021-3080-z
Shen, Y., Ni, W.-X., & Li, B. (2021). Porous Organic Polymer Synthesized by Green Diazo-Coupling Reaction for Adsorptive Removal of Methylene Blue. ACS Omega, 6(4), 3202-3208. https://doi.org/10.1021/acsomega.0c05634
Si, Y., & Samulski, E. T. (2008). Synthesis of Water Soluble Graphene. Nano Letters, 8(6), 1679-1682. https://doi.org/10.1021/nl080604h
Tajik, S., Beitollahi, H., Nejad, F. G., Dourandish, Z., Khalilzadeh, M. A., Jang, H. W., Venditti, R. A., Varma, R. S., & Shokouhimehr, M. (2021). Recent Developments in Polymer Nanocomposite-Based Electrochemical Sensors for Detecting Environmental Pollutants. Industrial & Engineering Chemistry Research, 60(3), 1112-1136. https://doi.org/10.1021/acs.iecr.0c04952
Thommes, M., Kaneko, K., Neimark, A. V., Olivier, J. P., Rodriguez-Reinoso, F., Rouquerol, J., & Sing, K. S. W. (2015). Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure and Applied Chemistry, 87(9-10), 1051-1069. https://doi.org/10.1515/pac-2014-1117
Trivedi, M. K., Branton, A., Trivedi, D., Nayak, G., Singh, R., & Jana, S. (2015). Characterization of physical, thermal and spectroscopic properties of Biofield Energy Treated p-Phenylenediamine and p-Toluidine. Environmental & Analytical Toxicology, 5(6), Artículo 1000329. https://doi.org/10.4172/2161-0525.1000329
Wang, J., He, J., Zhi, C., Luo, B., Li, X., Pan, Y., Cao, X., & Gu, H. (2014). Highly efficient synthesis of azos catalyzed by the common metal copper (0) through oxidative coupling reactions. RSC Advances, 4(32), 16607-16611. https://doi.org/10.1039/C4RA00749B
Wang, Z., He, M., Chen, B., & Hu, B. (2020). Azo-linked porous organic polymers/polydimethylsiloxane coated stir bar for extraction of benzotriazole ultraviolet absorbers from environmental water and soil samples followed by high performance liquid chromatography-diode array detection. Journal of Chromatography A, 1616, Artículo 460793. https://doi.org/10.1016/j.chroma.2019.460793
Wongsa, P., Phatikulrungsun, P., & Prathumthong, S. (2022). FT-IR characteristics, phenolic profiles and inhibitory potential against digestive enzymes of 25 herbal infusions. Scientific Reports, 12(1), Artículo 6631. https://doi.org/10.1038/s41598-022-10669-z
Zhang, T., Bai, R., Shen, J., Wang, Q., Wang, P., Yuan, J., & Fan, X. (2018). Laccase-catalyzed polymerization of diaminobenzenesulfonic acid for pH-responsive color-changing and conductive wool fabrics. Textile Research Journal, 88(19), 2258-2266. https://doi.org/10.1177/0040517517720497
Zhang, Y., Hong, X., Cao, X.-M., Huang, X.-Q., Hu, B., Ding, S.-Y., & Lin, H. (2021). Functional Porous Organic Polymers with Conjugated Triaryl Triazine as the Core for Superfast Adsorption Removal of Organic Dyes. ACS Applied Materials & Interfaces, 13(5), 6359-6366. https://doi.org/10.1021/acsami.0c21374
Zhang, Y., & Riduan, S. N. (2012). Functional porous organic polymers for heterogeneous catalysis. Chemical Society Reviews, 41(6), 2083-2094. https://doi.org/10.1039/C1CS15227K
Zhao, G., Huang, X., Tang, Z., Huang, Q., Niu, F., & Wang, X. (2018). Polymer-based nanocomposites for heavy metal ions removal from aqueous solution: A review. Polymer Chemistry, 9(26), 3562-3582. https://doi.org/10.1039/C8PY00484F
Zhao, J., Yan, G., Zhang, X., Feng, Y., Li, N., Shi, J., & Qu, X. (2022). In situ interfacial polymerization of lithiophilic COF@PP and POP@PP separators with lower shuttle effect and higher ion transport for high-performance Li–S batteries. Chemical Engineering Journal, 442, Artículo 136352. https://doi.org/10.1016/j.cej.2022.136352
Zhou, W., Yoshino, M., Kita, H., & Okamoto, K. (2001). Carbon Molecular Sieve Membranes Derived from Phenolic Resin with a Pendant Sulfonic Acid Group. Industrial & Engineering Chemistry Research, 40(22), 4801-4807. https://doi.org/10.1021/ie010402v
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2024 Byron Lopez Mayorga, Edward M. A. Guerrero-Gutiérrez, Allan Vásquez-Bolaños, José Castillo-Arroyave, Heriberto Pfeiffer

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
El autor que publique en esta revista acepta las siguientes condiciones:
- El autor otorga a la Dirección General de Investigación el derecho de editar, reproducir, publicar y difundir el manuscrito en forma impresa o electrónica en la revista Ciencia, Tecnología y Salud.
- La Direción General de Investigación otorgará a la obra una licencia Creative Commons Atribución-NoComercial-CompartirIgual 4.0 Internacional
Datos de los fondos
-
Dirección General de Investigación Científica y Técnica
Números de la subvención AP2-2022









