Colorimetric characterization of thermogravimetric process for dehydroxilation of hydrothermal and tuff kaolins
DOI:
https://doi.org/10.36829/63CTS.v9i1.924Keywords:
metakaolin, calcination, density, dehydroxylationAbstract
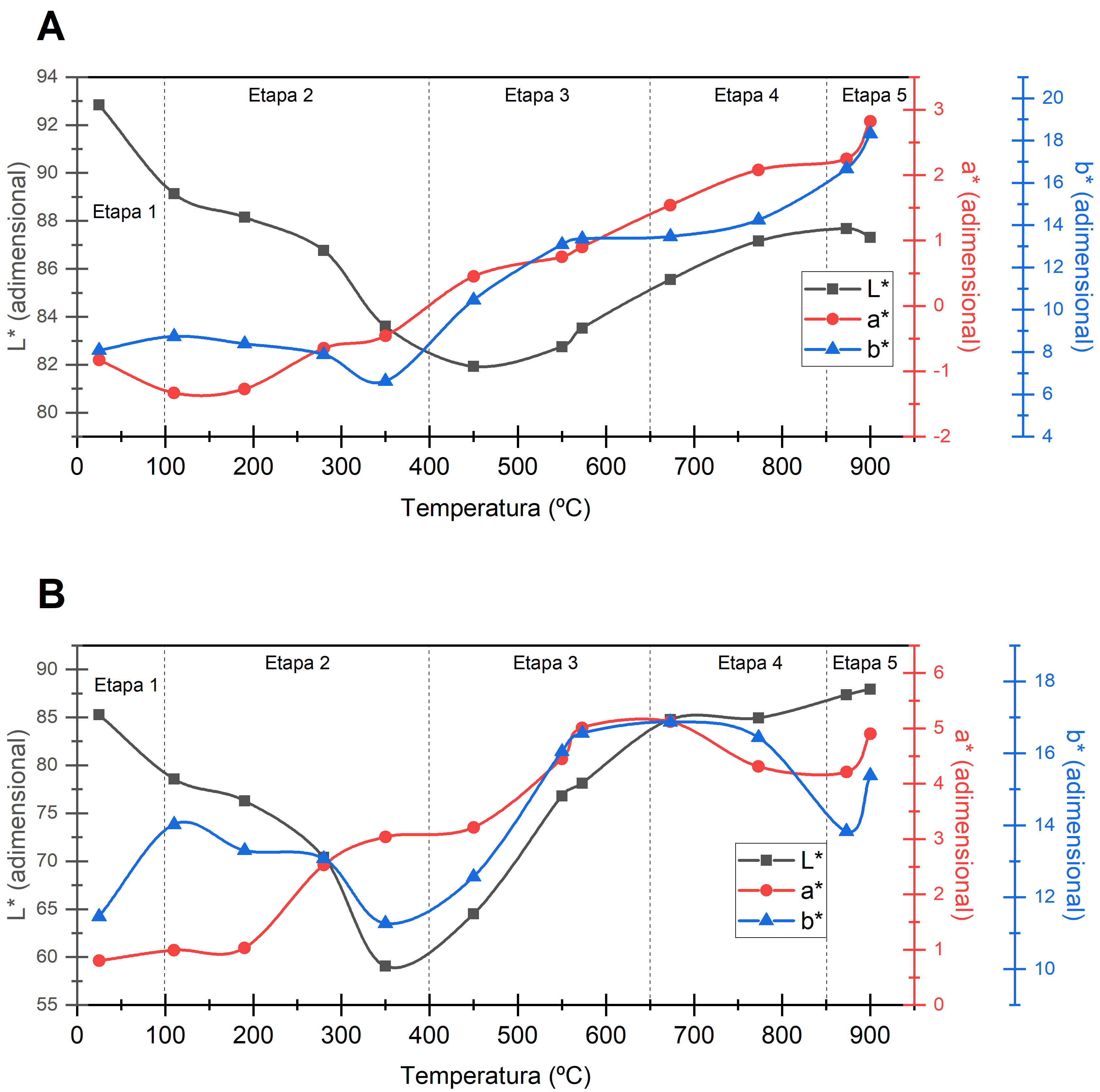
Metakaolin is a product of kaolin's calcination. The high pozzolanic activity of metakaolin allows its usage as supplementary cementitious material in concrete. This property and other physicochemical properties are affected by metakaolin's manufacturing conditions. Therefore, this study aims to characterize the changes in color and density of two types of kaolin (tuff and hydrothermal) through a thermogravimetric analysis of the calcination process. Evaluation of density used ASTM C188, while the assessment of color changes used a CIE-L*a*b* spectrophotometer in conjunction with normative UNE 80117. In addition, weight loss and density were correlated with the color coordinates using polynomial regression. The results showed that kaolin dehydroxylation occurred at 450ºC and 550ºC, characterized by a maximum in delta E * of 12.9 and 4.3 for hydrothermal and tuff kaolin, respectively. In addition, the tuff kaolin presented the maximum luminosity (L * = 92.84) of all the treatments at 21ºC. This value decreased 11.75% during the temperature increment up to 450ºC. From this temperature, L * increased linearly until reaching a final value of 87.3 at 900ºC. The polynomial regression explained 93% and 92% of the weight variation as a function of the CIE-L*a*b* parameters for tuff and hydrothermal kaolin, respectively.
Downloads
References
Ababneh, A., & Matalkah, F. (2018). Potential use of Jordanian volcanic tuffs as supplementary cementitious materials. Case Studies in Construction Materials, 8, 193-202. https://doi.org/10.1016/j.cscm.2018.02.004 DOI: https://doi.org/10.1016/j.cscm.2018.02.004
Badogiannis, E., Kakali, G., & Tsivilis, S. (2005). Metakaolin as supplementary cementitious material. Journal of Thermal Analysis & Calorimetry, 81(2), 457-462. https://doi.org/10.21809/rilemtechlett.2019.94 DOI: https://doi.org/10.1007/s10973-005-0806-3
Bellotto, M., Gualtieri, A., Artioli, G., & Clark, S. M. (1995). Kinetic study of the kaolinite-mullite reaction sequence. Part I: Kaolinite dehydroxylation. Physics and Chemistry of Minerals, 22(4), 207-217. https://doi.org/10.1007/BF00202253
https://doi.org/10.1007/BF00202253 DOI: https://doi.org/10.1007/BF00202253
Byrappa, K., & Yoshimura, M. (2013). 1 - Hydrothermal Technology-Principles and Applications.Elsevier. En Handbook of hydrothermal technology (Second Edition). https://doi.org/10.1016/B978-0-12-375090-7.00001-3 DOI: https://doi.org/10.1016/B978-0-12-375090-7.00001-3
Caballero, L. R., Macedo Paiva, M. das D., Fairbairn, E. de M. R., & Toledo Filho, R. D. (2019). Thermal, mechanical and microstructural analysis of metakaolin based geopolymers. Materials Research, 22(2). http://dx.doi.org/10.1590/1980-5373-mr-2018-0716 DOI: https://doi.org/10.1590/1980-5373-mr-2018-0716
Castelein, O., Soulestin, B., Bonnet, J. P., & Blanchart, P. (2001). The influence of heating rate on the thermal behaviour and mullite formation from a kaolin raw material. Ceramics International, 27(5), 517-522. https://doi.org/10.1016/S0272-8842(00)00110-3 DOI: https://doi.org/10.1016/S0272-8842(00)00110-3
Chandrasekhar, S., & Ramaswamy, S. (2006). Iron minerals and their influence on the optical properties of two Indian kaolins. Applied Clay Science, 33(3-4), 269-277. https://doi.org/10.1016/j.clay.2006.06.008 DOI: https://doi.org/10.1016/j.clay.2006.06.008
Chen, Y. F., Wang, M. C., & Hon, M. H. (2004). Phase transformation and growth of mullite in kaolin ceramics. Journal of the European Ceramic Society, 24(8), 2389-2397. https://doi.org/10.1016/S0955-2219(03)00631-9 DOI: https://doi.org/10.1016/S0955-2219(03)00631-9
Chen, Y., Zhou, C., Alshameri, A., Zhou, S., Ma, Y., Sun, T., Liang, H., Gong, Y., Wang, H., Yan, C. (2014). Effect of rice hulls additions and calcination conditions on the whiteness of kaolin. Ceramics International, 40(8, Part A), 11751-11758. https://doi.org/10.1016/j.ceramint.2014.04.003 DOI: https://doi.org/10.1016/j.ceramint.2014.04.003
Chotoli, F., Quarcioni, V., Soares de Lima, S., Ferreira, J., & Ferreira, G. (2015). Clay Activation and Color Modification in Reducing Calcination Process: Development in Lab and Industrial Scale. RILEM Bookseries, 10, 479-486. https://doi.org/10.1007/978-94-017-9939-3_59 DOI: https://doi.org/10.1007/978-94-017-9939-3_59
Fornasini, L., Bergamonti, L., Bondioli, F., Bersani, D., Lazzarini, L., Paz, Y., & Lottici, P. P. (2019). Photocatalytic N-doped TiO2 for self-cleaning of limestones. The European Physical Journal Plus, 134(10), Artículo 539. https://doi.org/10.1140/epjp/i2019-12981-6 DOI: https://doi.org/10.1140/epjp/i2019-12981-6
Frías, M., Rojas, M., Largo, O., García, R., Vigil, R., & Vegas, I. (2006). Viabilidad científica y técnica de reciclar metacaolín activado de residuos de lodos de papel como material puzolánico. Cemento y Hormigón, (893), 6-15.
Gagg, C. R. (2014). Cement and concrete as an engineering material: An historic appraisal and case study analysis. Engineering Failure Analysis, 40, 114-140. https://doi.org/10.1016/j.engfailanal.2014.02.004 DOI: https://doi.org/10.1016/j.engfailanal.2014.02.004
Gámiz, E., Melgosa, M., Sánchez-Marañón, M., Martín-García, J. M., & Delgado, R. (2005). Relationships between chemico-mineralogical composition and color properties in selected natural and calcined Spanish kaolins. Applied Clay Science, 28(1), 269-282. https://doi.org/10.1016/j.clay.2004.02.004 DOI: https://doi.org/10.1016/j.clay.2004.02.004
Gartner, E. (2004). Industrially interesting approaches to "low-CO2" cements. Cement and Concrete Research, 34(9), 1489-1498. https://doi.org/10.1016/j.cemconres.2004.01.021 DOI: https://doi.org/10.1016/j.cemconres.2004.01.021
Gilbert, P. U. P. A. (2022). Color-generating mechanisms. En Physics in the Arts (Chapter 9). Elsevier. https://doi.org/10.1016/B978-0-12-824347-3.00009-1 DOI: https://doi.org/10.1016/B978-0-12-824347-3.00009-1
Gualtieri, A., Bellotto, M., Artioli, G., & Clark, S. M. (1995). Kinetic study of the kaolinite-mullite reaction sequence. Part II: Mullite formation. Physics and Chemistry of Minerals, 22(4), 215-222. https://doi.org/10.1007/BF00202254 DOI: https://doi.org/10.1007/BF00202254
Guatame-García, A., Buxton, M., Deon, F., Lievens, C., & Hecker, C. (2018). Toward an on-line characterization of kaolin calcination process using short-wave infrared spectroscopy. Mineral Processing and Extractive Metallurgy Review, 39(6), 420-431. https://doi.org/10.1080/08827508.2018.1459617 DOI: https://doi.org/10.1080/08827508.2018.1459617
Haldar, S. K., & Tišljar, J. (2014). Chapter 5 - Sedimentary Rocks. En S. K. Haldar & J. B. T.-I. to M. and P. Tišljar, Eds.), Introduction to mineralogy and petrology (pp. 121-212). Elsevier. https://doi.org/10.1016/B978-0-12-408133-8.00005-5 DOI: https://doi.org/10.1016/B978-0-12-408133-8.00005-5
Hernández, A. C., Sánchez-Espejo, R., Meléndez, W., González, G., López-Galindo, A., & Viseras, C. (2019). Characterization of Venezuelan kaolins as health care ingredients. Applied Clay Science, 175, 30-39. https://doi.org/10.1016/j.clay.2019.01.003 DOI: https://doi.org/10.1016/j.clay.2019.01.003
Hunger, K. (1999). The effect of crystal structure on colour application properties of organic pigments. Review of Progress in Coloration and Related Topics, 29(1), 71-84. https://doi.org/10.1111/j.1478-4408.1999.tb00129.x DOI: https://doi.org/10.1111/j.1478-4408.1999.tb00129.x
Kenne Diffo, B. B., Elimbi, A., Cyr, M., Dika Manga, J., & Tchakoute Kouamo, H. (2015). Effect of the rate of calcination of kaolin on the properties of metakaolin-based geopolymers. Journal of Asian Ceramic Societies, 3(1), 130-138. https://doi.org/10.1016/j.jascer.2014.12.003 DOI: https://doi.org/10.1016/j.jascer.2014.12.003
Krajči, Ľ., Mojumdar, S., Janotka, I., Puertas, F., Palacios, M., & Kuliffayová, M. (2015). Performance of composites with metakaolin-blended cements. Journal of Thermal Analysis & Calorimetry, 119(2), 851-863. https://doi.org/10.1007/s10973-014-4119-2 DOI: https://doi.org/10.1007/s10973-014-4119-2
Liu, Y., Lei, S., Lin, M., Li, Y., Ye, Z., & Fan, Y. (2017). Assessment of pozzolanic activity of calcined coal-series kaolin. Applied Clay Science, 143, 159-167. https://doi.org/10.1016/j.clay.2017.03.038 DOI: https://doi.org/10.1016/j.clay.2017.03.038
López, A., Guzmán, G. A., & Di Sarli, A. R. (2016). Color stability in mortars and concretes. Part 1: Study on architectural mortars. Construction and Building Materials, 120, 617-622. https://doi.org/10.1016/j.conbuildmat.2016.05.133 DOI: https://doi.org/10.1016/j.conbuildmat.2016.05.133
López, A., & Sarli, A. R. Di. (2020). Measurements number in cementitious mixtures to define the color and its homogeneity. Construction and Building Materials, 238, Artículo 117636. https://doi.org/10.1016/j.conbuildmat.2019.117636 DOI: https://doi.org/10.1016/j.conbuildmat.2019.117636
Myers, R. H., Montgomery, D. C., & Anderson-Cook, C. M. (2011). Response surface methodology: Process and product optimization using designed experiments. Wiley.
Nedunuri, S. S. S. A., Sertse, S. G., & Muhammad, S. (2020). Microstructural study of Portland cement partially replaced with fly ash, ground granulated blast furnace slag and silica fume as determined by pozzolanic activity. Construction and Building Materials, 238, Artículo 117561. https://doi.org/10.1016/j.conbuildmat.2019.117561 DOI: https://doi.org/10.1016/j.conbuildmat.2019.117561
Nmiri, A., Hamdi, N., Yazoghli-Marzouk, O., Duc, M., & Srasra, E. (2017). Synthesis and characterization of kaolinite-based geopolymer: Alkaline activation effect on calcined kaolinitic clay at different temperatures. Journal of Materials and Environmental Sciences, 8(2), 676-690.
Păcurariu, C., Lazău, I., & Lazău, R. (2017). Kinetic studies of the dehydroxylation and crystallization of raw kaolinite and fluorides-modified kaolinite. Journal of Thermal Analysis & Calorimetry, 127(1), 239-246. https://doi.org/10.1007/s10973-016-5763-5 DOI: https://doi.org/10.1007/s10973-016-5763-5
Ptáček, P., Šoukal, F., Opravil, T., Havlica, J., & Brandštetr, J. (2011). The kinetic analysis of the thermal decomposition of kaolinite by DTG technique. Powder Technology, 208(1), 20-25. https://doi.org/10.1016/j.powtec.2010.11.035 DOI: https://doi.org/10.1016/j.powtec.2010.11.035
Ramli, M., & Alonge, O. R. (2016). Characterization of metakaolin and study on early age mechanical strength of hybrid cementitious composites. Construction and Building Materials, 121, 599-611. https://doi.org/10.1016/j.conbuildmat.2016.06.039 DOI: https://doi.org/10.1016/j.conbuildmat.2016.06.039
Restrepo Gutiérrez, J. C., Restrepo Baena, O. J., & Tobón, J. I. (2006). Efectos de la adición de metacaolín en el cemento pórtland. Dyna, 73(150), 131-141.
Smiley, W. D., Bartich, G., Stromgberg, M., Lemley, R., Antram, R. L., & Snider, K. T. (1998). Method of producing metakaolin (U.S. Patent No. US5792251A). U.S. Patent and Trademark Office. https://patft.uspto.gov/netacgi/nph-Parser?Sect2=PTO1&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch-bool.html&r=1&f=G&l=50&d=PALL&RefSrch=yes&Query=PN%2F5792251
Ssegane, H., Tollner, E. W., Mohamoud, Y. M., Rasmussen, T. C., & Dowd, J. F. (2012). Advances in variable selection methods I: Causal selection methods versus stepwise regression and principal component analysis on data of known and unknown functional relationships. Journal of Hydrology, 438-439, 16-25. https://doi.org/10.1016/j.jhydrol.2012.01.008 DOI: https://doi.org/10.1016/j.jhydrol.2012.01.008
Tanwongwan, W., Wongkitikun, T., Onpecht, K., Srilai, S., Assabumrungrat, S., Chollacoop, N., & Eiad-ua, A. (2020). Surface enhancement and structure formation of metakaolin from thailand kaolin on the various calcination temperature. Materials Today: Proceedings, 23(part 4), 777-781. https://doi.org/10.1016/j.matpr.2019.12.273 DOI: https://doi.org/10.1016/j.matpr.2019.12.273
Tironi, A., Trezza, M. A., Scian, A. N., & Irassar, E. F. (2012). Kaolinitic calcined clays: Factors affecting its performance as pozzolans. Construction and Building Materials, 28(1). https://doi.org/10.1016/j.conbuildmat.2011.08.064 DOI: https://doi.org/10.1016/j.conbuildmat.2011.08.064
Torres, J., de Gutiérrez, R. M., Castelló, R., & Vizcayno, C. (2011). Análisis comparativo de caolines de diferentes fuentes para la producción de metacaolín. La Revista Latinoamericana de Metalurgia y Materiales, 31(1), 35-43.
Trusilewicz, L., Fernández-Martínez, F., Rahhal, V., Talero, R., & Jantzen, C. (2012). TEM and SAED characterization of metakaolin. Pozzolanic activity. Journal of the American Ceramic Society, 95(9), 2989-2996. https://doi.org/10.1111/j.1551-2916.2012.05325.x DOI: https://doi.org/10.1111/j.1551-2916.2012.05325.x
Uzal, B., & Turanli, L. (2003). Studies on blended cements containing a high volume of natural pozzolans. Cement and Concrete Research, 33(11), 1777-1781. https://doi.org/10.1016/S0008-8846(03)00173-X DOI: https://doi.org/10.1016/S0008-8846(03)00173-X
Valanciene, V., Siauciunas, R., & Baltusnikaite, J. (2010). The influence of mineralogical composition on the colour of clay body. Journal of the European Ceramic Society, 30(7), 1609-1617. https://doi.org/10.1016/j.jeurceramsoc.2010.01.017 DOI: https://doi.org/10.1016/j.jeurceramsoc.2010.01.017
Walpole, R. E., Myers, R. H., Myers, S. L., & Ye, K. (2012). Probability & statistics for engineers & scientists (9th. ed.). Pearson.
Wang, H., Li, C., Peng, Z., & Zhang, S. (2011). Characterization and thermal behavior of kaolin. Journal of Thermal Analysis & Calorimetry, 105(1), 157-160. https://doi.org/10.1007/s10973-011-1385-0 DOI: https://doi.org/10.1007/s10973-011-1385-0
White, C. E., Provis, J. L., Proffen, T., Riley, D. P., & van Deventer, J. S. J. (2010a). Combining density functional theory (DFT) and pair distribution function (PDF) analysis to solve the structure of metastable materials: The case of metakaolin. Physical Chemistry Chemical Physics, 12(13), 3239-3245. https://doi.org/10.1039/B922993K DOI: https://doi.org/10.1039/b922993k
White, C. E., Provis, J. L., Proffen, T., Riley, D. P., & van Deventer, J. S. J. (2010b). Density functional modeling of the local structure of kaolinite subjected to thermal dehydroxylation. The Journal of Physical Chemistry A, 114(14), 4988-4996. https://doi.org/10.1021/jp911108d DOI: https://doi.org/10.1021/jp911108d
Xie, N. (2016). Mechanical and environmental resistance of nanoparticle-reinforced pavement materials. En K. J. Loh & S. Nagarajaiah (Eds.), Innovative developments of advance multifunctional nanocomposites in civil and structural engineering (pp. 217-246). Elsevier. https://doi.org/10.1016/B978-1-78242-326-3.00010-5 DOI: https://doi.org/10.1016/B978-1-78242-326-3.00010-5
Yu, L. (2002). Color Changes Caused by Conformational Polymorphism: Optical-crystallography, single-crystal spectroscopy, and computational chemistry. The Journal of Physical Chemistry A, 106(3), 544-550. https://doi.org/10.1021/jp013019c DOI: https://doi.org/10.1021/jp013019c
Zemenová, P., Kloužková, A., Kohoutková, M., & Král, R. (2014). Investigation of the first and second dehydroxylation of kaolinite. Journal of Thermal Analysis & Calorimetry, 116(2), 633-639. https://doi.org/10.1007/s10973-014-3748-9 DOI: https://doi.org/10.1007/s10973-014-3748-9
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Giorgio E. A. López-Pardo, César A. García-Guerra, Roberto Lainfiesta, Edward M. A. Guerrero-Gutiérrez

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
El autor que publique en esta revista acepta las siguientes condiciones:
- El autor otorga a la Dirección General de Investigación el derecho de editar, reproducir, publicar y difundir el manuscrito en forma impresa o electrónica en la revista Ciencia, Tecnología y Salud.
- La Direción General de Investigación otorgará a la obra una licencia Creative Commons Atribución-NoComercial-CompartirIgual 4.0 Internacional









