Evaluación de la producción de cuerpos fructíferos de cepas guatemaltecas del hongo comestible Rukoxil Tunay Che’ (Agrocybe cylindracea (DC.: Fr. ) Maire) en diferentes sustratos
DOI:
https://doi.org/10.36829/63CTS.v1i1.8Resumen
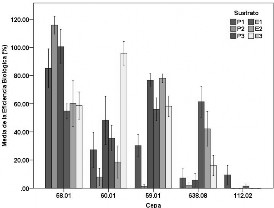
En el presente estudio se determinó la producción de cuerpos fructíferos de cinco cepas nativas de Agrocybe cylindracea sobre tres sustratos y dos tratamientos térmicos, a través del porcentaje de eficiencia biológica y la medición del diámetro de los píleos. Se encontró que el mayor porcentaje de eficiencia biológica de las cepas en los sustratos evaluados fue 115.84 %, que correspondió al sustrato constituido por 29% paja de trigo mas 1% de harina de soya pasteurizado y obtenido por la cepa 58.01, la cual también produjo las mayores eficiencias biológicas en todos los sustratos evaluados. Al confrontar el porcentaje de eficiencia biológica en los diferentes sustratos, todas las cepas presentaron valores altos en el sustrato compuesto por 28% de paja de trigo, 1% harina de soya y CaCO3. Con respecto al diámetro de los cuerpos fructíferos, las cepas 58.01, 59.01, 60.01 y 638.08 produjeron píleos menores de 2 cm, entre 2-4 cm y mayores a 4.0 cm en los diferentes sustratos y tratamientos, excepto la cepa 59.01 que en el sustrato formulado con 29% de paja de trigo y 1% de harina de soya, solo produjo cuerpos fructíferos con píleos menores a 2 cm y entre 2-4 cm. En el análisis proximal de los basidiomas de las cepas evaluadas se obtuvo un alto porcentaje de proteínas, fibra cruda y carbohidratos, así como bajo porcentaje de grasas.
Descargas
Citas
Andrade, C. (2007). Descripción de las características de cultivo in vitro de cepas nativas de Agrocybe aegerita (Brigant) Singer. (Tesis de graduación: Química Bióloga) Universidad de San Carlos de Guatemala, Facultad de Ciencias Químicas y Farmacia, Guatemala.
Bateman J. (1970). Nutrición animal. Manual de mé- todos analíticos. México D.F: Centro Regional de Ayuda Técnica.
Bran, M., Morales, O., Cáceres, R. y Flores, R. (2003a). Contribución al conocimiento de los hongos comestibles de Guatemala. Revista Científica, 1(1), 2-24.
Bran, M., Morales, O., Cáceres. R., y Flores, R. (2003b). Hongos comestibles de Guatemala: Diversidad, cultivo y nomenclatura vernácula. (Fase III). (Inf-2003-30). Universidad de San Carlos de Guatemala, Dirección General de Investigación, Guatemala.
Bran, M., Morales, O., Flores, R., Cáceres, R. y Gurriarán, N. (2009). Cultivo de cepas guatemaltecas del hongo comestible Tx'yol B'aqman (Agrocybe cylindracea (DC.) Maire): caracterización y producción de cuerpos fructíferos. (Inf-2009-45). Universidad de San Carlos de Guatemala, Dirección General de Investigación, Guatemala.
Chang S., & Miles P. (2004). Mushrooms cultivation, nutritional value, medicinal effect, and environmental impact. (2nd. ed.). Boca Raton: CRC Press.
Coello-Castillo, M., Sánchez, J., & Royse, D. (2009). Production of Agaricus bisporus on substrates pre-colonizated by Scytalidium thermophilum and supplemented at casing with protein-rich supple- ments. Bioresource Technology, 100, 4488-4492. https://doi.org/10.1016/j.biortech.2008.10.061
De León, R., Lau, D., Vallejo, R. y Klee, C. (2012). Requerimientos fisiológicos que inciden en el crecimiento micelial y la degradación del sustrato por Agrocybe aegerita. En J. Sánchez y G. Mata (Eds), Hongos comestibles y medicinales en Iberoamérica, investigación y desarrollo en un entorno multicultural (pp. 241-254). México: ECOSUR-INECOL.
Kües, U., & Liu, Y. (2000). Fruiting body production in basidiomycetes. Applied Microbiology and Bio- technology, 54, 141-152. https://doi.org/10.1007/s002530000396
Labarére, J. y Bois, F. (2001). La conservación y el uso de los recursos genéticos de Pleurotus spp. En J. Sanchez, y D. Royse. (Eds),. La biología y el cultivo de Pleurotus spp. (p. 83-123). México D.F.: Limusa.
Lechner, B., & Albertó, E. (2011). Search for new naturally occurring strains of Pleurotus to improve yields. Pleurotus albidus as a novel proposed species for mushroom cultivation. Revista Iberoamericana de Micología, 28(4), 148-154. https://doi.org/10.1016/j.riam.2010.12.001
Luang, R., Liang, Y., Chen, Y., Liu, H., Jiang, S., Che, T., & Sun, H. (2010). Opposing developmental functions of Agrocybe aegerita galectin (AAL) during mycelia differentiation. Fungal Biology II, 4, 599-608. https://doi.org/10.1016/j.funbio.2010.05.001
Madigan, M., Martinko, J., Dunlap, P. y Clark, D. (2009). Brock Biología de los microorganismos. (12ª. ed.). Madrid: Pearson.
Morales, O., Bran, M. y Cáceres, R. (2010). Los hongos comestibles de uso tradicional en Guatemala. En D. Martínez-Carrera, N. Curvetto, M. Sobal, P. Morales, y V. Mora. (Eds). Hacia un Desarrollo Sostenible del Sistema de Producción-Consumo de los Hongos Comestibles y Medicinales en La- tinoamérica: Avances y Perspectivas en el Siglo XXI. (p. 437-464). Puebla: COLPOS-UNSCO- NACYT-AMC-UAEM-UPAEP-IMINAP.
Official Methods of Analysis of AOAC International. (2000) 17th ed. Gaithersburg: AOAC International. Omarini, A., Lechner, B., & Albertó, E. (2009). Polyporus tenuiculus: a new naturally ocurring mushroom that can be industrially cultivated on agricultural waste. Journal of Industrial Microbiology & Biotechnology, 36, 635-642. https://doi.org/10.1007/s10295-009-0530-2
Quimio, T., & Chang, S. (1990). Technical guidelines for mushroom growing in the tropics. Roma: Food and Agriculture Organization.
Tsai, S., Tsai, H., & Mau, J. (2008). Non-volatile taste components of Agaricus blazei, Agrocybe cylindracea and Boletus edulis. Food Chemistry, 107, 977-983. https://doi.org/10.1016/j.foodchem.2007.07.080
Uhart, M., & Albertó, E. (2007). Morphologic characterization of Agrocybe cylindracea (Basidiomycetes, Agaricales) from America, Europe and Asia. Revista Mexicana de Micología, 24, 9-18.
Uhart, M., & Albertó, E., (2009). Mating test in Agrocybe cylindracea sensu lato, recognition of Agrocybe wrightii as a novel species. Mycological Progress, 8, 337-349. https://doi.org/10.1007/s11557-009-0607-3
Uhart, M., Piscera, J., & Albertó, E. (2008). Utilization of new ocurring strains and supplementation to improve the biological efficiency of the edible mushroom Agrocybe cylindracea. Journal of Industrial Microbiology and Biotechnology, 35, 595-602. https://doi.org/10.1007/s10295-008-0321-1
Wood, D. (1985). Production and roles of extracellular enzymes during morphogenesis of basidiomycete fungi. In D. Moore, L. Casselton, D. Wood, & J. Frankland, (Eds), Developmental biology of higher fungi (p. 375-387). Cambridge: Cambridge University Press.
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2014 María del Carmen Bran, Roberto Cáceres, Natalia Gurriarán, Osberth Morales, Roberto Flores

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
El autor que publique en esta revista acepta las siguientes condiciones:
- El autor otorga a la Dirección General de Investigación el derecho de editar, reproducir, publicar y difundir el manuscrito en forma impresa o electrónica en la revista Ciencia, Tecnología y Salud.
- La Direción General de Investigación otorgará a la obra una licencia Creative Commons Atribución-NoComercial-CompartirIgual 4.0 Internacional









