Using software to evaluate thermodynamics properties of pure substances in thermodynamics courses
DOI:
https://doi.org/10.36829/63CTS.v10i2.1603Keywords:
Free Open source, Jupyter notebook, CoolProp, Cantera, teaching thermodynamics, PythonAbstract
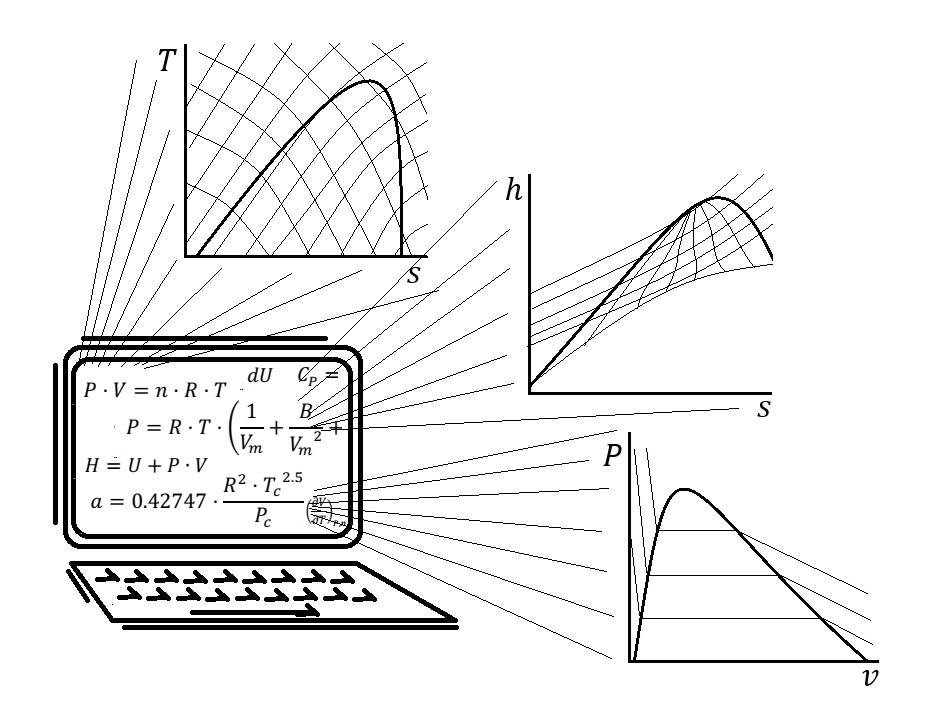
Is in thermodynamics courses where engineering students learn how to estimate the thermodynamic properties of substances, by using different kinds of models including equations of state, thermodynamics tables, and charts. The thermodynamics tables are the result of tabulating the information obtained by using multiparameter equations, the information that is shown in these tables could also be saved in different kinds of software. The implementation of this kind of technology in a thermodynamics course brings different advantages, like saving time spent on calculations and allowing to focus the class on the study of concepts. However, the economic value associated with purchasing user licenses could be a limitation to use this kind of software. To avoid this problem the implementation of free open source software could be evaluated. Examples of this kind of software are the libraries of thermodynamic data: Cantera and CoolProp, which can be accessed by using different programming languages like Python. The use of Cantera and CoolProp is free. When using this kind of software to estimate data the user must write programming codes. This may seem intimidating for a person with little experience in coding, but this could also be an advantage because it could lead the student to understand the relationship between the variables, the thermodynamic concepts, and the codes needed in his calculations. Is in thermodynamics courses where engineering students learn how to estimate the thermodynamic properties of substances, by using different kinds of models including equations of state, thermodynamics tables, and charts. The thermodynamics tables are the result of tabulating the information obtained by using multiparameter equations, the information that is shown in these tables could also be saved in different kinds of software. The implementation of this kind of technology in a thermodynamics course brings different advantages, like saving time spent on calculations and allowing to focus the class on the study of concepts. However, the economic value associated with purchasing user licenses could be a limitation to the use of this kind of software. To avoid this problem the implementation of free open source software could be evaluated. Examples of this kind of software are the libraries of thermodynamic data: Cantera and CoolProp, which can be accessed by using different programming languages like Python. The use of Cantera and CoolProp is free. When using this kind of software to estimate data the user must write programming codes. This may seem intimidating for a person with little experience in coding, but this could also be an advantage because it could lead the student to understand the relationship between the variables, the thermodynamic concepts, and the codes needed in his calculations.
Downloads
References
Bakrania, S., & Mallouk, K. (2017). Blowing off Steam Tables. 2017 ASEE Annual Conference & Exposition Proceedings. https://doi.org/10.18260/1-2--27661
Bell, I. H., Wronski, J., Quoilin, S., & Lemort, V. (2014). Pure and pseudo-pure fluid thermophysical property evaluation and the open-source thermophysical property library CoolProp. Industrial & Engineering Chemistry Research, 53(6), 2498-2508. https://doi.org/10.1021/ie4033999
Castier, M., & Amer, M. M. (2011). XSEOS: An evolving tool for teaching chemical engineering thermodynamics. Education for Chemical Engineers, 6(2), Artículo e62-e70. https://doi.org/10.1016/j.ece.2010.12.002
Çengel, Y. A., & Boles, M. A. (2015). Properties of pure substances. En Thermodynamics: An engineering approach (8th ed., pp. 124-134). McGraw-Hill.
ChemicaLogic Corporation. (2003). Thermodynamic and transport properties of water and steam (2.0). ChemicaLogic Corporation. http://www.chemicalogic.com/Pages/DownloadSteamTabCompanion.html
Craig, P. A., Nash, J. A., & Crawford, T. D. (2022). Python scripting for biochemistry and molecular biology in Jupyter Notebooks. Biochemistry and Molecular Biology Education, 50(5), 479-482. https://doi.org/10.1002/bmb.21676
F-chart Software. (2023). Engineering Equation Solver (11.620 2023-06-11). https://fchartsoftware.com/ees/
Goodwin, D. G., Moffat, H. K., Schoegl, I., Speth, R. L., & Weber, B. W. (2022). Cantera: An object-oriented software toolkit for chemical kinetics, thermodynamics, and transport processes (2.6.0). Zenodo. https://doi.org/https://doi.org/10.5281/zenodo.6387882
Gourde, R. M., & Akih-Kumgeh, B. (2017). A Matlab program for the determination of thermodynamic properties of steam. International Journal of Mechanical Engineering Education, 45(3), 228-244. https://doi.org/10.1177/0306419016682146
Granger, B. E., & Pérez, F. (2021). Jupyter: Thinking and torytelling with ode and Data. Computing in Science & Engineering, 23(2), 7-14. https://doi.org/10.1109/MCSE.2021.3059263
Harvey, A. H., & Burgess, D. R. (2021). Fifty years of reference data. Journal of Physical and Chemical Reference Data, 50(1), Artículo 010401. https://doi.org/10.1063/5.0040316
Kazakov, A., Muzny, C. D., Chirico, R. D., Diky, V. V., & Frenkel, M. (2008). Web thermo tables - an on-line version of the TRC Thermodynamic Tables. Journal of Research of the National Institute of Standards and Technology, 113(4), 209. https://doi.org/10.6028/jres.113.016
Kim, C., Kim, H., & Mun, K. (2020). Use of the international association for the properties of water and steam (IAPWS) formulations, IAPWS-95 & IAPWS-IF97: Making of Mollier diagram and T-s diagram of water and steam. Thermal Science and Engineering Progress, 20, Artículo 100691. https://doi.org/10.1016/J.TSEP.2020.100691
Lehtola, S., & Karttunen, A. J. (2022). Free and open source software for computational chemistry education. WIREs Computational Molecular Science, 12(5). https://doi.org/10.1002/wcms.1610
Lemmon, E. W., Bell, I. H., Huber, M. L., & McLinden, M. O. (2018). NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-(REFPROP) Version 10. National Institute of Standards and Technology, Standard Reference Data Program. https://doi.org/https://doi.org/10.18434/T4/1502528
Lide, D. R. (1996). SteamTab: Thermodynamic and transport properties of steam. Journal of Chemical Information and Computer Sciences, 36(6), 1228-1228. https://doi.org/10.1021/ci960123w
Linstrom, P. J., & Mallard, W. G. (2001). The NIST Chemistry WebBook: A chemical data resource on the internet. Journal of Chemical & Engineering Data, 46(5), 1059-1063. https://doi.org/10.1021/je000236i
Linstrom, P. J., & Mallard, W. G. (Eds.). (2023). NIST chemistry WebBook, NIST Standard Reference Database Number 69. National Institute of Standards and Technology. https://doi.org/https://doi.org/10.18434/T4D303
Liu, Y. (2011). Development of instructional courseware in thermodynamics education. Computer Applications in Engineering Education, 19(1), 115-124. https://doi.org/10.1002/cae.20297
Martin, C. R., Moore, J. P., & Ranalli, J. A. (2016). Teaching the foundations of thermodynamics with PYro. 2016
IEEE Frontiers in Education Conference (FIE), 1-6. https://doi.org/10.1109/FIE.2016.7757589
Mayhew, Y. R. (1991). Does the methodology of teaching thermodynamics to engineers need changing for the 1990s? Proceedings of the Institution of Mechanical Engineers, Part A: Journal of Power and Energy, 205(4), 283-286. https://doi.org/10.1243/PIME_PROC_1991_205_038_02
MegaWatSoft. (s.f.). Steam97 Application. MegaWatSoft. Rcuperado el 11 de junio de 2023, de https://www.megawatsoft.com/steam-tables/steam97-application.aspx
Mulop, N., Yusof, K. M., & Tasir, Z. (2012). A review on enhancing the teaching and learning of thermodynamics. Procedia - Social and Behavioral Sciences, 56, 703-712. https://doi.org/10.1016/j.sbspro.2012.09.706
Nehra, V., & Tyagi, A. (2014). Free open source software in electronics engineering education: A survey. International Journal of Modern Education and Computer Science, 6(5), 15-25. https://doi.org/10.5815/ijmecs.2014.05.03
Perkel, J. M. (2015). Programming: Pick up Python. Nature, 518(7537), 125-126. https://doi.org/10.1038/518125a
Reades, J. (2020). Teaching on Jupyter. Region, 7(1), 21-34. https://doi.org/10.18335/region.v7i1.282
Rolon-Mérette, D., Ross, M., Rolon-Mérette, T., & Church, K. (2020). Introduction to Anaconda and Python: Installation and setup. The Quantitative Methods for Psychology, 16(5), S3-S11. https://doi.org/10.20982/tqmp.16.5.S003
Smith, J. M., Van Ness, H. C., Abbot, M. M., & Swihart, M. T. (2018). A ppendix E. Steam tables. En Introduction to chemical engineering thermodynamics (8th ed., pp. 684-723). McGraw-Hill.
Southard, M. Z., Rowley, R. L., & Wilding, W. V. (2019). Physical and Chemical Data. En D. W. Green & M. Z. Southard (Eds.), Perry’s chemical engineers’ handbook (9th ed., pp. 191-265). McGraw-Hill.
Span, R. (2000). Multiparameter equations of state: An accurate source of thermodynamic property data. Springer Science & Business Media. https://doi.org/10.1007/978-3-662-04092-8
Vallejo, W., Díaz-Uribe, C., & Fajardo, C. (2022). Google Colab and virtual simulations: Practical e-learning tools to support the teaching of thermodynamics and to introduce coding to students. ACS Omega, 7(8), 7421-7429. https://doi.org/10.1021/acsomega.2c00362
Wagner, W., & Pruß, A. (2002). The IAPWS formulation 1995 for the thermodynamic properties of ordinary water substance for general and scientific Use. Journal of Physical and Chemical Reference Data, 31(2), 387-535. https://doi.org/10.1063/1.1461829
Wang, Y., Li, M., Wang, X.-S., Gildersleeve, A., & Turki, N. (2023). ATRP Kinetic Simulator: An online open resource educational tool using Jupyter Notebook and Google colaboratory. Journal of Chemical Education, 100(7), 2770-2775. https://doi.org/10.1021/acs.jchemed.2c01250
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Ana Rufina Herrera Soto, William E. Fagiani

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
El autor que publique en esta revista acepta las siguientes condiciones:
- El autor otorga a la Dirección General de Investigación el derecho de editar, reproducir, publicar y difundir el manuscrito en forma impresa o electrónica en la revista Ciencia, Tecnología y Salud.
- La Direción General de Investigación otorgará a la obra una licencia Creative Commons Atribución-NoComercial-CompartirIgual 4.0 Internacional









