Perfil de citocinas Th1, Th2, Th17 y otras citocinas pro inflamatorias (IL-1β, IL-6 y TNFα) en el plasma de pacientes con cáncer gástrico
DOI:
https://doi.org/10.36829/63CTS.v8i2.1071Palabras clave:
Tumor gástrico, biomarcadores plasmáticos en cáncer, respuesta inmune en cáncer gástrico, marcadores tumorales, diagnóstico de cáncer gástricoResumen
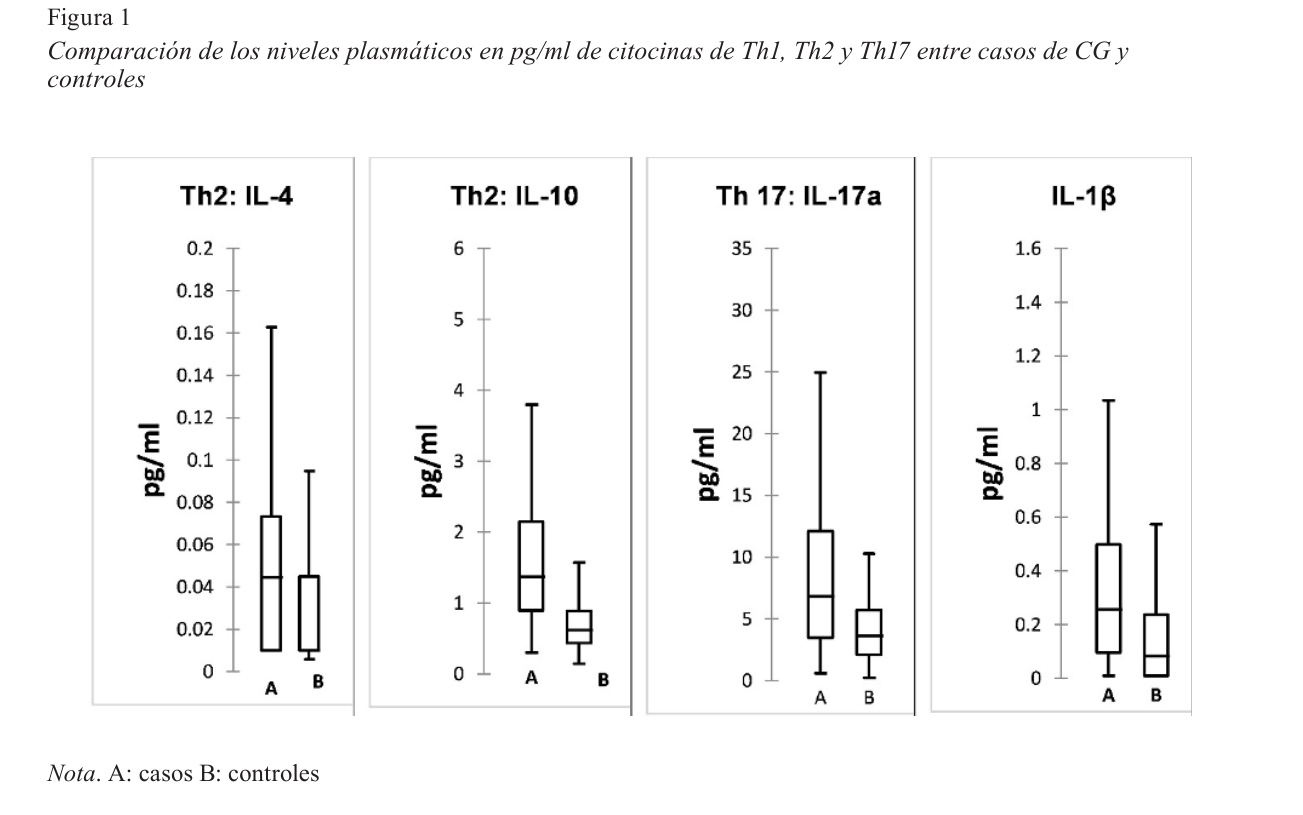
El cáncer gástrico (CG) es la neoplasia del tubo digestivo más prevalente en el mundo, asociada a factores genéticos del hospedero y externos, como infección por Helicobacter pylori. La patogénesis incluye inflamación crónica mediada por citocinas del microambiente tumoral, detectables sistémicamente. Estudios previos reportan niveles séricos de citocinas y su contribución al diagnóstico de CG. El presente estudio analiza el perfil de citocinas del tipo de Th1(IFNγ), Th2(IL-4 e IL-10), Th17(Th-17A) y otras pro inflamatorias: IL-1β, IL-6 y TNF-α, en plasma de 70 casos de pacientes con CG comparándolos con 132 sujetos sanos equiparables en edad y sexo. Los casos provinieron del Hospital Roosevelt e Instituto Nacional de Cancerología de Guatemala (Incan) y formaron parte de un estudio previo. Se analizó la base de datos clínicos, patológicos y epidemiológicos. Se midieron los niveles de citocinas utilizando el sistema “MSD MULTI-SPOT Assay System”. La edad promedio de los casos fue 59.5 años, (DE 13.0), 51%, eran positivos para IgG anti H. pylori. Un 71% presentó adenocarcinoma grado III (Borrman), según clasificación de Lauren 55% tenían tipo intestinal. Las siete citocinas cuantificadas se encontraron significativamente elevadas (p < .05) en el plasma de los casos respecto a sus controles. Los casos de CG tipo difuso presentaron niveles de IFNγ significativamente elevados. Por regresión logística, las citocinas IL-6 e IL-10, están asociadas significativamente a CG (p < .05) independientemente del estatus de infección por H. pylori. Se destacan la IL-6 e IL-10 como las principales citocinas asociadas a la presencia de CG.
Descargas
Citas
Abbasi, A., & Heydari, S. (2018). Studying the expression rate and methylation of Reprimo gene in the blood of patients suffering from gastric cancer. European journal of Ttranslational Myology, 28(2), 7423. https://doi.org/10.4081/ejtm.2018.7423 DOI: https://doi.org/10.4081/ejtm.2018.7423
Alpízar-Alpízar, W., Pérez-Pérez, G. I., Une, C. P., Cuenca, & Sierra, R. (2005). Association of interleukin-1B and interleukin-1RN polymorphisms with gastric cancer in a high-risk population of Costa Rica. Clinical Experimental Medicine, (5), 169-176. https://doi.org/10.1007/s10238-005-0082-3 DOI: https://doi.org/10.1007/s10238-005-0082-3
Alpízar-Alpízar, W., Une C., & Sierra R. (2009). La inflamación y su papel en el desarrollo del cáncer gástrico. Acta Médica Costarricense, 51(2), 76-81. DOI: https://doi.org/10.51481/amc.v51i2.224
Amieva, M., & Peek, R. (2016) Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology, 150(1), 64-78. https://doi.org/10.1053/j.gastro.2015.09.004. DOI: https://doi.org/10.1053/j.gastro.2015.09.004
Ashizawa, T., Okada, R., Suzuki, Y., Takagi, M., Yamazaki, T., Sumi, T., Oki, T., Ohnuma, S., & Aoki, T. (2005). Clinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: Role of IL-6 as a prognostic factor. Gastric Cancer, 8(2),124-131. https://doi.org/10.1007/s10120-005-0315-x. DOI: https://doi.org/10.1007/s10120-005-0315-x
Bednarz-Misa, I., Diakowska, D., Szczuka, I., Fortuna, P., Kubiak, A., Rosińczuk, J., & Krzystek-Korpacka, M. (2020). Interleukins 4 and 13 and their Receptors are differently expressed in gastrointestinal tract cancers, depending on the anatomical site and disease advancement, and improve colon cancer cell viability and motility. Cancers, 12(6), 1463. https://doi.org/10.3390/cancers12061463 DOI: https://doi.org/10.3390/cancers12061463
Cárdenas, D. M., Sánchez, A. C., Rosas, D. A., Rivero, E., Paparoni, M. D., Cruz, M. A., Suárez, Y. P., & Galvis, N. F., (2018). Preliminary analysis of single-nucleotide polymorphisms in IL-10, IL-4, and IL-4Rα genes and profile of circulating cytokines in patients with gastric Cancer. BMC Gastroenterol, 18, Article 184. https://doi.org/10.1186/s12876-018-0913-9 DOI: https://doi.org/10.1186/s12876-018-0913-9
Catalano, V., Labianca, R., Beretta, G. D., Gatta, G., Braud, F., & Cutsem, E. (2009). Gastric cancer. Critical Review in Oncology/Hematology, 71(2), 127-164. https://doi.org/10.1016/j.critrevonc.2009.01.004 DOI: https://doi.org/10.1016/j.critrevonc.2009.01.004
Chiurillo, M. A. (2014). Role of gene polymorphisms in gastric cancer and its precursor lesions: Current knowledge and perspectives in Latin American countries. World Journal of Gastroenterology, 20(16), 4503-4515. https://doi.org/10.3748/wjg.v20.i16.4503 DOI: https://doi.org/10.3748/wjg.v20.i16.4503
Coomes, E. A., & Haghbayan, H. (2020). Interleukin-6 in Covid-19: A systematic review and meta-analysis. Reviews in Medical Virology, 30(6), 1-9. https://doi.org/10.1002/rmv.2141 DOI: https://doi.org/10.1002/rmv.2141
Correa, P. (1992). Human gastric carcinogenesis: A multistep and multifactorial process First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Research, 52(24), 6735-6740.
Cover, T., & Blanke, S. (2005). Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nature Reviews Microbiology, 3, 320-332. https://doi.org/10.1038/nrmicro1095. DOI: https://doi.org/10.1038/nrmicro1095
Cover, T. (2016). Helicobacter pylori diversity and gastric cancer risk. American Society of Microbiology, 7(1), 1-9. https://doi.org/10.1128/mBio.01869-15. DOI: https://doi.org/10.1128/mBio.01869-15
El-Omar, E. M., Carrington, M., Chow, W. H., McColl, K. E., Bream, H. J., Young, H. A., Herrera, J., Lissow, J., Youan, C., Rothman, N., Lanyon, G., Martin, M. Fraulmeni Jr., J., & Rabkin, C. S. (2000). Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature, 404(6842), 398-402. https://doi.org/10.1038/35006081 DOI: https://doi.org/10.1038/35006081
Epplein, M., Xiang, Y.-B., Cai, Q., Peek Jr., R. M., Li, H., Correa, P., Gao, J., Wu, J., Michel, A., Pawlita, M., Zheng, W., & Shu, X.-O. (2013). Circulating cytokines and gastric cancer risk. Cancer Causes & Ccontrol, 24(12), 2245-2250. https://doi.org/10.1007/s10552-013-0284-z DOI: https://doi.org/10.1007/s10552-013-0284-z
Ferlay, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., & Bray, F. (2015). Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer, 136(5), E359-E386. https://doi.org/.1002/ijc.29210 DOI: https://doi.org/10.1002/ijc.29210
Fernández-Botrán, R., Wellmann, I. A., Une, C., Méndez-Chacón, E., Hernández de Rodas, E., Bhandari, B., & Villagrán de Tercero, C. I. (2020). Seroprevalence of Helicobacter pylori CagA Antibodies in Guatemalan Gastric Cancer Patients: Association of Seropositivity with Increased Plasma Levels of Pepsinogens but not Soluble Urokinase Plasminogen Activator Receptor. The American Journal of Tropical Medicine and Hygiene, 103(1), 260-265. https://doi.org/10.4269/ajtmh.19-0934 DOI: https://doi.org/10.4269/ajtmh.19-0934
Franceschi, C., Capri, M., Monti, D., Giunta, S., Olivieri, F., Sevini, F., Panourgia, M. P., Invidia, L., Celani, L., Scurti, M., Cevenini, E., Castellani, G. C., & Salvioli, S. (2007). Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mechanisms of Ageing and Development, 128(1), 92-105. https://doi.org/10.1016/j.mad.2006.11.016 DOI: https://doi.org/10.1016/j.mad.2006.11.016
Foran, E., Garrity-Park, M. M., Mureau, C., Newell, J., Smyrk, T. C., Limburg, P. J., & Egan, L. J. (2010). Upregulation of DNA methyltransferase-mediated gene silencing, anchorage-independent growth, and migration of colon cancer cells by interleukin-6. Molecular Cancer Research: MCR, 8(4), 471-481. https://doi.org/10.1158/1541-7786.MCR-09-0496 DOI: https://doi.org/10.1158/1541-7786.MCR-09-0496
Hansen, S., Vollset, S. E., Derakhshan, M. H., Fyfe, V., Melby, K. K., Aase, S., Jellum, E., & McColl, K. E. (2007). Two distinct aetiologies of cardia cancer; evidence from premorbid serological markers of gastric atrophy and Helicobacter pylori status. Gut, 56(7), 918-925. DOI: https://doi.org/10.1136/gut.2006.114504
Herrera-Goepfer, R., Zúñiga, J., Hernández-Guerrero, A., Rodríguez-Reyna, T., Osnalla, N., Ruíz-Morales, J., Vargas-Alarcón, G., Yamamoto-Furusho, J., Mohar- Betancourt, A., Hernández-Pando, R., & Granados, J., (2004). Asociación del alelo HLA-DQB1*0501 del complejo mayor de histocompatibilidad con cáncer gástrico en México. Gaceta Médica de México, 140(3), 299-303.
Hitzler, I., Sayi, A., Kohler, E., Engler, D. B., Koch, K. N., Hardt, W.-D., & Müller, A. (2012). Caspase-1 has both proinflammatory and regulatory properties in Helicobacter infections, which are differentially mediated by its substrates IL-1β and IL-18. Journal of Immunology (Baltimore, Md.: 1950), 188(8), 3594-3602. https://doi.org/10.4049/jimmunol.1103212 DOI: https://doi.org/10.4049/jimmunol.1103212
Imai, Y., Chiba, T., Kondo, T., Kanzaki, H., Kanayama, K., Ao, J., Kojima, R., Kusakabe, Y., Nakamura, M., Saito, T., Nakagawa, R., Suzuki, E., Nakamoto, S., Muroyama, R., Tawada, A., Matsumura, T., Nakagawa, T., Kato, J., Kotani, A., … Kato, N. (2020). Interferon-γ induced PD-L1 expression and soluble PD-L1 production in gastric cancer. Oncology Letters, 20(3), 2161-2168. https://doi.org/10.3892/ol.2020.11757 DOI: https://doi.org/10.3892/ol.2020.11757
Gao, J.-F.,Zhang, H. Lv, J., Wang, L., & Fan, Y.-Y. (2019). Associations of the IL-17A rs2275913 and IL-17F rs763780 polymorphisms with the risk of digestive system neoplasms: A meta-analysis. International Immunopharmacology, 67, 248-259. https://doi.org/10.1016/j.intimp.2018.12.016
Ju, X., Zhang, H., Zhou, Z., Chen, M., & Wang, Q. (2020). Tumor-associated macrophages induce PD-L1 expression in gastric cancer cells through IL-6 and TNF-ɑ signaling. Experimental Cell Research, 396(2), 112315. https://doi.org/10.1016/j.yexcr.2020.112315 DOI: https://doi.org/10.1016/j.yexcr.2020.112315
Jie-Fang Gao, Hong Zhang, Jian Lv, Li Wang, Yue-Ying Fan, (2019) Associations of the IL-17A rs2275913 and IL-17F rs763780 polymorphisms with the risk of digestive system neoplasms: A meta-analysis, International Immunopharmacology, 67, 248-259, https://doi.org/10.1016/j.intimp.2018.12.016. DOI: https://doi.org/10.1016/j.intimp.2018.12.016
Kao, J. Y., Rathinavelu, S., Eaton, K. A., Bai, L., Zavros, Y., Takami, M., Pierzchala, A., & Merchant, J. L. (2006). Helicobacter pylori-secreted factors inhibit dendritic cell IL-12 secretion: a mechanism of ineffective host defense. American Journal of Physiology. Gastrointestinal and Liver Physiology, 291(1), G73-G81. https://doi.org/10.1152/ajpgi.00139.2005 DOI: https://doi.org/10.1152/ajpgi.00139.2005
Karabulut, M., Usul Afsar, C., Serimez, M., & Karabulut, S. (2019). Serum IL-17 levels can be diagnostic for gastric cancer. Journal of B.U.ON.: Official Journal of the Balkan Union of Oncology, 24(4), 1601-1609.
Kulmambetova, G., Shtefanov, I., Aitkulova, A., Imanbekova, M., Iskakova, A., Makishev, A., & Ramankulov, Y. (2020). Association of polymorphisms in TP53 and the promoter region of IL10 with gastric cancer in a Kazakh population. Bosnian journal of Basic Medical Sciences, 20(4), 539-546. https://doi.org/10.17305/bjbms.2020.4761 DOI: https://doi.org/10.17305/bjbms.2020.4761
Landskron, G., De la Fuente, M., Thuwajit, P., Thuwajit, C., & Hermoso, M. A. (2014). Chronic inflammation and cytokines in the tumor microenvironment. Journal of Immunology Research, Article 19149185. https://doi.org/10.1155/2014/149185 DOI: https://doi.org/10.1155/2014/149185
Lindholm, C., Quiding-Järbrink, M., Lönroth, H., Hamlet, A., & Svennerholm, A. M. (1998). Local cytokine response in Helicobacter pylori-infected subjects. Infection and Immunity, 66(12), 5964-5971. https://doi.org/10.1128/IAI.66.12.5964-5971.1998 DOI: https://doi.org/10.1128/IAI.66.12.5964-5971.1998
Long, Z.-W., Yu, H.-M., Wang, Y.-N., Liu, D., Chen, Y.-Z., Zhao, Y.-X., & Bai, L. (2015). Association of IL-17 polymorphisms with gastric cancer risk in Asian populations, World Journal of Gastroenterology, 21(18), 5707-5718. https://doi.org/10.3748/wjg.v21.i18.5707 DOI: https://doi.org/10.3748/wjg.v21.i18.5707
Malfertheiner, P., Megraud, F. O’Morain, C. A. O., Gisbert, J. P., Kuipers, E. J., Axon, A. T., Bazzoli, F., Gasbarrini, A. Atherton, J. Graham, D. Y. Hunt, R. Moayyedi, P., Rokkas, T., Rugge, M., Selgrad, M., Suerbaum, S., Sugano, K., & El-Omar, E. M. (2017). Management of helicobacter pylori infection-the maastricht V/Florence consensus report. Gut, 66(1), 6-30. https://doi.org/10.1136/gutjnl-2016-312288 DOI: https://doi.org/10.1136/gutjnl-2016-312288
Peek, R. M., Fiske, & C., & Wilson, K. T. (2010). Role of innate immunity in Helicobacter pylori-induced gastric malignancy. Physiological Reviews, 90(3), 831-858. https://doi.org/10.1152/physrev.00039.2009 DOI: https://doi.org/10.1152/physrev.00039.2009
Petrovich, I., & Ford, J.M. (2016). Genetic predisposition to gastric cancer. Seminars in Oncology, 43(5), 554-559. https://doi.org/10.1053/j.seminoncol.2016.08.006. DOI: https://doi.org/10.1053/j.seminoncol.2016.08.006
Poorolajal, J., Moradi, L., Mohammadi, Y., Cheraghi, Z., & Gohari-Ensaf, F. (2020). Risk factors for stomach cancer: A systematic review and meta-analysis. Epidemiology and Health, 42, Article e2020004. https://doi.org/10.4178/epih.e2020004 DOI: https://doi.org/10.4178/epih.e2020004
Saavedra, H., & García Verdecia, B. (2014). Inmunosenescencia: Efectos de la edad sobre el sistema inmune. Revista Cubana de Hematología, Inmunología y Hemoterapia, 30(4), 332-345.
Sánchez-Zauco, N., Torres, J., Gómez, A., Camorlinga-Ponce, M., Muñoz-Pérez, L., Herrera-Goepfert, Medrano-Guzmán, Giono-Cerezo, S., & Maldonado-Bernal, C. (2017). Circulating blood levels of IL-6, IFN- γ, and IL-10 as potential diagnostic biomarkers in gastric cancer: A controlled study. BMC Cancer, 17(1), Article 384, https://doi.org/10.1186/s12885-017-3310-9 DOI: https://doi.org/10.1186/s12885-017-3310-9
Shokrzadeh, M., Mohmmadpour, A., Hoseini, V., Abediankenari, S., Ghassemi-Barghin, N., & Tabari, Y. (2018). Serum cytokine of IL-2, IL-10 and IL-12 levels in patients with stomach adenocarcinoma. Arquivos de Gastroenterologia, 55(4) 385-389. https://doi.org//10.1590/s0004-2803.201800000-83. DOI: https://doi.org/10.1590/s0004-2803.201800000-83
Sung, H., Ferlay, J., Siegel, R., Laversanne, M., Soerjomataram, I., Jemal, A., Bray, F. (2020) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer Journal for Clinicians, 71(1),7-33. https://doi.org/10.3322/caac.21660 DOI: https://doi.org/10.3322/caac.21660
Szkaradkiewicz, A., Karpiński, T. M., Drews, M., Borejsza-Wysocki, M., Majewski, P., Andrzejewska, E. (2010). Natural killer cell cytotoxicity and immunosuppressive cytokines (IL-10, TGF-beta1) in patients with gastric cancer. Journal of Biomedicine & Biotechnology, Article 901564. https://doi.org/10.1155/2010/901564. DOI: https://doi.org/10.1155/2010/901564
Torres, M. M., Acosta, C. P., Sicard, D. M., & Groot de Restrepo, H. (2004). Susceptibilidad genética y riesgo de cáncer gástrico en una población del Cauca. Biomedica, 24(2), 153-62. DOI: https://doi.org/10.7705/biomedica.v24i2.1261
Udomsinprasert, W., Jittikoon, J., Sangroongruangsri, S., & Chaikledkaew, U. (2021). Circulating levels of interleukin-6 and interleukin-10, but not tumor necrosis factor-Alpha, as potential biomarkers of severity and mortality for COVID-19: Systematic Review with Meta-analysis. Journal of Clinical Immunology, 41(1), 11-22. https://doi.org/10.1007/s10875-020-00899-z DOI: https://doi.org/10.1007/s10875-020-00899-z
Vahid, F., & Davoodi, S. H. (2021). Nutritional Factors Involved in the Etiology of Gastric Cancer: A Systematic Review. Nutrition and Cancer, 73(3), 376-390. https://doi.org/10.1080/01635581.2020.1756353 DOI: https://doi.org/10.1080/01635581.2020.1756353
van den Engel, N. K., Winter, H., Rüttinger, D., Shau, I., Schiller, M., Mayer, B., Moudgil, T., Meimarakis, G., Stolte, M., Jauch, K. W., Fox, B. A., & Hatz, R. A. (2006). Characterization of immune responses in gastric cancer patients: A possible impact of H. pylori to polarize a tumor-specific type 1 response? Clinical Immunology, 120(3), 285-296. https://doi.org/10.1016/j.clim.2006.04.566 DOI: https://doi.org/10.1016/j.clim.2006.04.566
Wellmann, I. A., Villagrán, C. I., Fernández-Botrán R., Hernández, E., & Une, C. (2018). Valor diagnóstico de las proteínas uPAR en sangre para el cáncer gástrico en Guatemala.Ciencia Tecnología y Salud, 5(1), 43-53. https://doi.org/10.36829/63CTS.v3i2.341 DOI: https://doi.org/10.36829/63CTS.v5i1.478
Zhai, Z., Zhu, Z.-Y., Zhang, Y., Yin, X., Han, B.-L., Gao, J.-L., Lou, S- H., Fang, T.-Y., Wang, Y.-M., Li, C.-F., Yu, X.-F., Ma, Y., & Xue, Y.-W. (2020). Prognostic significance of Borrmann type combined with vessel invasion status in advanced gastric cancer. World Journal of Gastrointestinal Oncology, 12(9), 992-1004. https://doi.org/10.4251/wjgo.v12.i9.992 DOI: https://doi.org/10.4251/wjgo.v12.i9.992
Zhang, W.-H., Wang, X.-L., Zhou, J., An, L.-Z., Xie, X.-D. (2005). Association of interleukin-1B (IL-1B) gene polymorphisms with risk of gastric cancer in Chinese population. Cytokine, 30(6), 378-381. https://doi.org/10.1016/j.cyto.2005.02.002 DOI: https://doi.org/10.1016/j.cyto.2005.02.002
Zhao, S., Wu, D., Wu, P., Wang, Z., & Huang, J. (2015). Serum IL-10 Predicts Worse Outcome in Cancer Patients: A Meta-Analysis. PloS One, 10(10), Article e0139598. https://doi.org/10.1371/journal.pone.0139598 DOI: https://doi.org/10.1371/journal.pone.0139598
Zhou, X., Zhu, H., Zhu, C., Lin, K., Cai, Q., Li, Z., & Du, Y. (2020). Helicobacter pylori infection and serum pepsinogen level with the risk of gastric Precancerous Conditions: A Cross-sectional study of high-risk gastric cancer population in China. Journal of Clinical Gastroenterology, 55(9), 778-784. https://doi.org/10.1097/MCG.0000000000001444 DOI: https://doi.org/10.1097/MCG.0000000000001444
Zou, Z., Zhao, L., Su, S., Liu, Q., Yu, L., Wei, J., Yang, Y., Juan, D., Jie, S., Xiaoping, Q.,
Xiangshan, F., Wenxian, G., Baorui, L., (2018). The plasma levels of 12 cytokines and growth factors in patients with gastric cancer. Medicine (Baltimore), 97(19), Article e0413. https://doi.org/10.1097/MD.0000000000010413 DOI: https://doi.org/10.1097/MD.0000000000010413
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2021 Carmen Villagran, Rafael Fernández-Botrán, Elisa Hernandez, Federico Nave, Irmgardt A. Wellmann, Jose F. Muñoz-Valle

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
El autor que publique en esta revista acepta las siguientes condiciones:
- El autor otorga a la Dirección General de Investigación el derecho de editar, reproducir, publicar y difundir el manuscrito en forma impresa o electrónica en la revista Ciencia, Tecnología y Salud.
- La Direción General de Investigación otorgará a la obra una licencia Creative Commons Atribución-NoComercial-CompartirIgual 4.0 Internacional









